OVERVIEW
Reinforcement corrosion is the most serious cause of deterioration of concrete structures which has the effect of reducing service life. Traditional approaches to dealing with deteriorating concrete involved making changes to the concrete design embedding the reinforcement, or adding more concrete cover rather than opting for corrosion resistant reinforcement.
There are many types of corrosion which can impact the steel; however, choosing the appropriate type of SSR for the service conditions should guarantee a full extended service life with minimal maintenance and repair.
There is a number of stainless steel reinforcement types provided in ASTM A955 which allows the Owner/designer a degree of latitude in custom fitting the type of rebar to the specific service conditions. However, to leverage the “custom fitting” advantage afforded by a choice of stainless alloys, a guidance format is required to select the appropriate type of stainless.
Such a guidance format for assessing where stainless steel reinforcement should be used and the selection of the appropriate type of material for the application is provided in the following sections. Some background material to this format is provided beforehand on the various types of corrosion impacting SSR, on a standard measure of corrosion, and a summary of the results of laboratory research on corrosion of SSR.
Corrosion defined
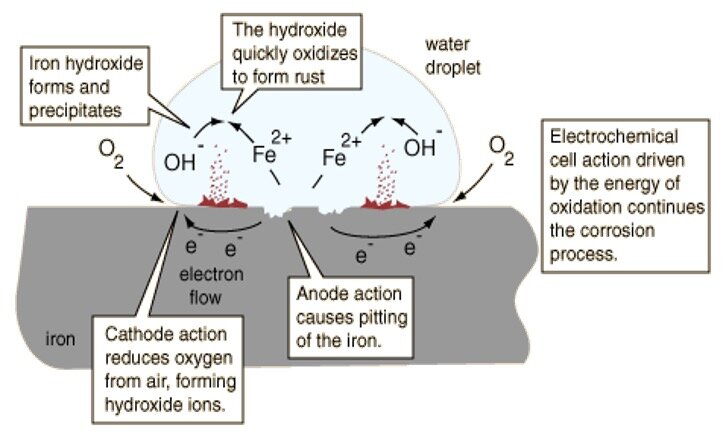
Corrosion is the gradual destruction of materials (usually metals) by chemical and/or electrochemical reaction with their environment. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen. Rusting which is the formation of iron oxide, is a well-known example of electrochemical corrosion.
Corrosion can also occur in materials other than metals, such as ceramics or polymers, or composites of polymer (such as GFRP) although in this context, the term "degradation" is more common.
As a consequence of corrosion, the useful properties of materials and structures including strength, appearance and permeability to liquids can be reduced.
Corrosion can extend across a wide area more or less uniformly corroding the surface or can be concentrated locally to form a pit or crack. Given that corrosion occurs on exposed surfaces, methods to reduce the corrosion activity of the exposed surface by means of passivation can increase a material's corrosion resistance.
Types of Corrosion
Corrosion of stainless steel reinforcement can be separated into two categories based on the type of corrosion medium :
“Wet corrosion” or corrosion in liquid or humid environments
Corrosion that occurs in hot gases at high temperatures above 500°C
Our focus is on corrosion in liquid or humid environments which includes atmospheric humidity. This type of metal corrosion involves an electrochemical process with an anode and cathode in the same electrolytic solution which connects the two poles. The metal oxidizes at the anode producing electrons which are consumed by the cathode reduction chemical reactions. For this process of electrochemical corrosion of stainless to occur the passivity layer supported by the chromium oxide layer would have to be compromised.
The information in Table 1 regarding the types of corrosion to which stainless steel is subjected and the necessary ambient conditions for corrosion pertains to the condition whereby the metal is exposed directly to the wet media and not in concrete.
Table 1
Corrosion Types / Necessary Conditions for Corrosion
/ Stainless Performance
|
Type of Corrosion |
Corrosion Defined |
Necessary conditions for corrosion |
Stainless Performance |
|
Uniform |
Uniform or general corrosion is a type of corrosion attack or deterioration that is uniformly distributed over the entire exposed surface of a metal. |
For uniform corrosion to occur the passive layer would have to be destroyed over the entire surface of the metal bar. This phenomenon is associated more with the normal deterioration process of carbon steel and is less severe than in stainless steel. |
This form of corrosion is more likely to occur on SSR exposed directly to highly acidic or hot alkaline environments rather than for stainless steel embedded in concrete. |
|
Pitting |
Pitting is highly localized corrosion with discrete pits on the steel surface. |
The factors that can cause pitting include a damaged or weak passive layer due to deposits of scale, corrosion products, weld oxides, excess dirt, etc. These foreign materials will cut off the oxygen necessary for re-passivation at the deposit location. |
Measures and standards are in place in the fabrication shops to ensure that the material is clean and free from all deposits. |
|
Crevice |
This type is a form of localized corrosion which is evident in crevices and confined spaces created by metal to metal contact. |
The necessary conditions that lead to crevice corrosion are improper design of metal to metal components with gaskets or joints with flanges and threaded connections which can be exacerbated by deposits of foreign materials on the surface of the stainless steel. The flow of oxygen to the tight crevice is limited by geometry and the shielding effect of deposits which weakens the passive layer facilitating corrosion. |
Measures and standards are in place in the fabrication shops to ensure that the material is clean and free from all deposits. In addition, the amount of surface area of stainless steel rebar material represented by potentially problematic connection components such as flanged or bolted joints or welded connections in the average project is insignificant. |
|
Galvanic |
The corrosion effect of connecting two dissimilar metals. |
The conditions include the presence of dissimilar metals that are electrically connected in a common electrolyte. The degree to which corrosion occurs is dependent upon the extent of the dissimilarity of the metals in terms of their position on the galvanic series, the strength of the electrolyte, the time for both metals to be exposed to the electrolyte, the relative surface areas of the metals. |
The research indicates that the galvanic coupling current induced by the connecting stainless steel to carbon steel is much lower than a carbon-carbon steel mix. In the presence of chlorides, a stainless-carbon connection produces a level of coupling current which is 2 to 3 times less than the carbon-carbon steel combination. |
|
Stress Corrosion Cracking (SCC) |
The formation of cracks by a combination of the effects of stress, corrosion and temperature. |
The normal conditions to induce cracking are high levels of tensile stress, active wet chloride corrosion and elevated temperatures (>60°C) levels. Research (HSE RR902) indicates other factors which may cause SCC include steel made with high levels of impurities, surface finish issues, iron contamination or deposits on the material, residual effects from welding, manufacturing components (gaskets, flanges) where chlorides can accumulate.
|
Under normal service conditions of stainless steel embedded in concrete it is not highly stressed by design, the designer presumably would have selected a type of stainless steel resistant to corrosion and the service temperatures would be below 30°C.
Modern steel making process virtually eliminates the possibility of any impurities in the steel, any issues with the surface finish and contamination/deposits are eliminated given the fabrication standards that are in place at the rebar plants, the material is not welded and the incidence of the use of manufacturing components is low. |
|
Intergranular Corrosion |
The reduction of corrosion resistance in the grain boundaries |
This type of corrosion occurs when the steel is heated to elevated temperatures (>427°C) during or before service which causes the precipitation of chromium carbides at the grain boundaries which reduces the corrosion resistance in the chromium deleted areas.
|
Stainless steel is not heated to these critical temperatures levels after the mill manufacturing stage. Also, this type of corrosion was a potential risk for high carbon content steels (0.05-0.15%). The carbon content of most of the commonly used types of steel today is in the range of 0.03-0.04%. |